MICROFLUIDICS IS NOW JUST
1-CLICK AWAY WITH NETRI SHOP
Discover our new exclusive package
organs-on-chip kits and all our
neuro-organs-on-chip devices.

Innervate. Screen. Predict.
Innervate any organ using neurons as a natural biosensor.
Screen acute or chronic responses using high throughut electrophysiological recording.
Predict clinical outcomes using AI.
• The genesis of NETRI
• Towards a revolution in the regulatory environment
• Towards a revolution in pharmaceutical research
• Organs-on-chip, in practice
• NETRI, industrial start-up
Based on 10 years of academic research, NETRI was founded in June 2018 in Lyon at the heart of the Gerland biodistrict. Since 2018, NETRI has developed a unique value proposition on the organs-on-chip market. NETRI solutions offer to generate the level of data (throughput) and biological complexity (relevance) required to validate the predictive nature of compounds in humans, with 3 main areas of differentiation:
• High-throughput & interoperable devices: compartmentalization, pump-free and compatible with laboratory equipment.
• Using neurons as a bio-digital sensors: digital transcription of the functional impact (determination of toxicity or efficacy) of products tested on an innervated target organ, creating a digital signature for each compound tested.
• Digital library: comparison of the digital signatures of the compound to be tested with those of compounds already on the market or which have failed in the clinical phase (prediction of toxicity and efficacy).
The marketing of new treatments, whatever their field of application, requires testing of their toxicity and efficacy on physiological models prior to regulatory approval. The dermo-cosmetics, pharmaceutical and food health markets in particular are concerned by this regulatory framework. However, these three markets are not subject to the same regulatory constraints when it comes to bringing new molecules to market:
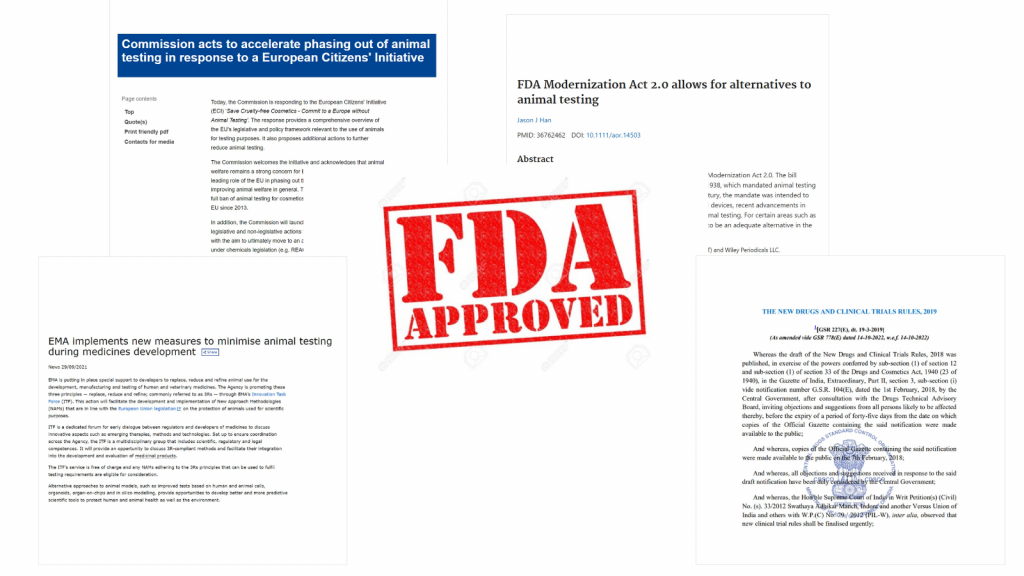
• The pharmaceutical industry is strictly regulated by regulatory authorities such as the FDA (Food Drug & Administration, USA), the EMA (European Medicines Agency, European Union) and ANSM (Agence Nationale de Sécurité du Médicament, France). Today, there is a fundamental trend towards speeding up accreditation processes, limiting the associated economic risks, and at the same time helping to limit the use of animal models in the pharmaceutical industry, as shown by recent actions by the FDA, as well as government initiatives in India and Europe.
• The other two segments, less constrained but more easily criticized from an ethical point of view, are ahead of the game when it comes to evaluating ingredients for creams or food agents. For a decade now, the dermo-cosmetics industry in Europe and most other countries has no longer been able to use animals in pre-marketing trials. The health food industry, for its part, will no longer be able to use animals from 2030.
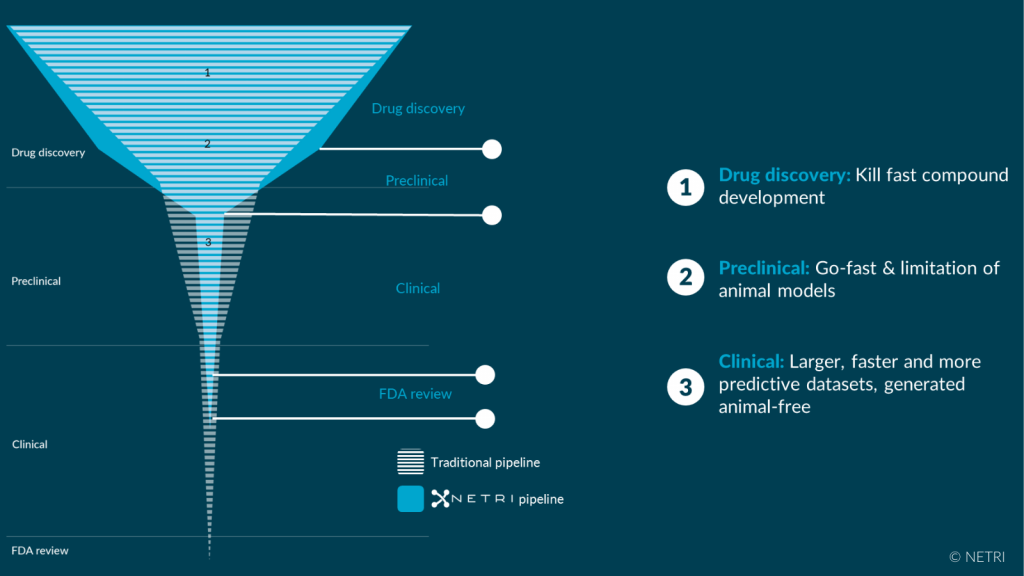
Healthcare industries developing treatments are looking for solutions to three structural challenges:
• Accelerate discovery and pre clinical research phases which consume patent application time.
• Reduce the failure rate in clinical phases (85% today).
• Minimize animal experimentation under pressure from politicians and public opinion in particular.
NETRI is convinced that the future of drug trials will focus on the rapid, replicable generation of large volumes of biological data (for artificial intelligence processing), generated with the increased translation offered by organs-on-chip (OoCs) , helping to limit animal experimentation. In this context, NETRI addresses the dermo-cosmetics, pharmaceutical and food health markets to meet their specific challenges.
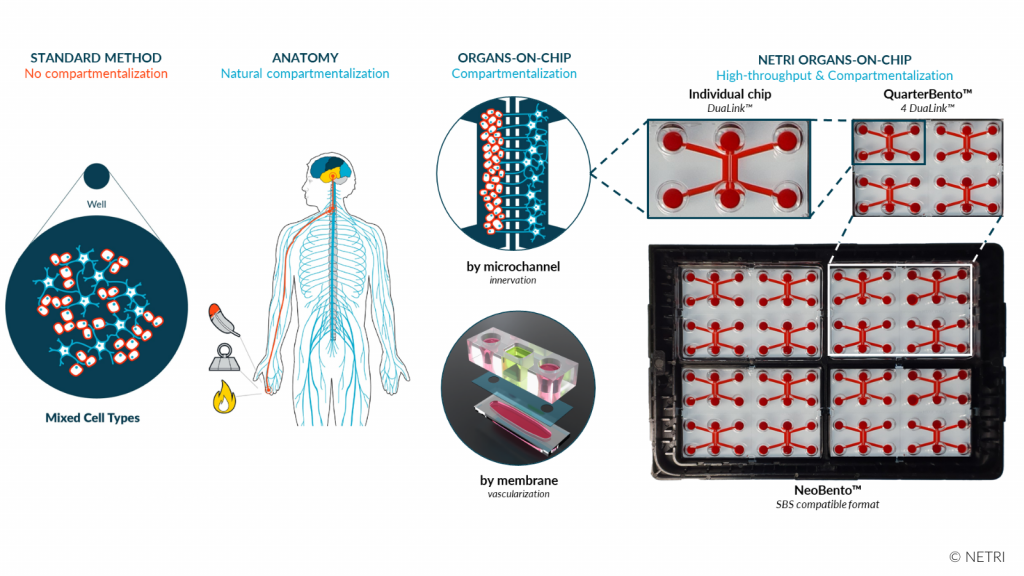
Organs-on-chip (OoCs) are microfluidic devices that enable human cells to be positioned and connected in the same way as in a healthy or diseased human body.
Organs-on-chip, imagined in 2011 by Professor Don Inberg and his lung-on-chip model, simulate the physiology of human body organs such as the brain, liver and heart. Historically positioned on neurological disorders, NETRI has enabled its customers to observe, through its technologies, curative effects of their molecules previously impossible to observe with conventional technologies.
By varying the connection architectures, it is possible to monitor, by pathology, the response of the neuronal network to new treatments by recording how neurons communicate with each other and with other cell types. NETRI positions itself within its ecosystem and in relation to its competitors as the only player enabling the innervation and vascularization of any organ, constituting a competitive advantage and a strategic barrier to entry for its direct competitors.

After 4 years of research and development, NETRI has built solutions using neurons as sensors integrated into its high-throughput, interoperable solutions, enabling the construction of a digital library of references. These solutions are offered in the form of ready-to-use devices (dry devices or kits), services and SaaS software:
• 8 devices marketed. The NeuroFluidics™ line, marketed since September 2022, comprising 4 devices is the best-selling due to the generic nature of its potential applications. The NeuroFluidics™ MEA line, marketed since March 2023 and developed with the support of Axion BioSystems (world leader in the market for neural activity recording equipment), is composed of 3 devices.
• 3 models marketed in 2 market segments. The innervated skin model, launched in September 2022 with BASF, targeting the dermo-cosmetics market. The pain-on-chip model, launched in June 2023, targeting the oncology segment of the pharmaceutical industry.
• 1 software, UpLink, marketed since March 2023 alongside the NeuroFluidics™ MEA line, which enables NETRI to interpret data recorded by neurons in devices, constituting a gateway to the digital library.
Discover our new exclusive package
organs-on-chip kits and all our
neuro-organs-on-chip devices.

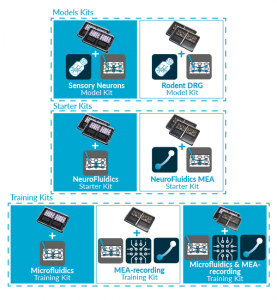
ORGANS-ON-CHIP KITS
Quickly and easily adopt organs-on-chip
into users’ research