MICROFLUIDICS IS NOW JUST
1-CLICK AWAY WITH NETRI SHOP
Discover our new exclusive package
organs-on-chip kits and all our
neuro-organs-on-chip devices.

Since 2018 , our experts have designed and manufactured pumpless organs-on-chip devices and models with a user-centric experience for a fast adoption of alternative methods in the regulatory approved process.
• Our Business
• What is microfluidic?
• What are organs-on-chip?
• Our approach

We focus on building partnerships that go beyond commercial alliances and provide innovative combined solutions to our industrial partners.
• Consumables with NETRI Products (NeuroFluidics Devices & NeuroFluidics Cultures)
• Softwares with NETRI Devices (NeuroFluidics Digital)
• Services & Licensing with NETRI Services, BioPharma & Sense (FTE R&D, FTE Services and Screnning Services)
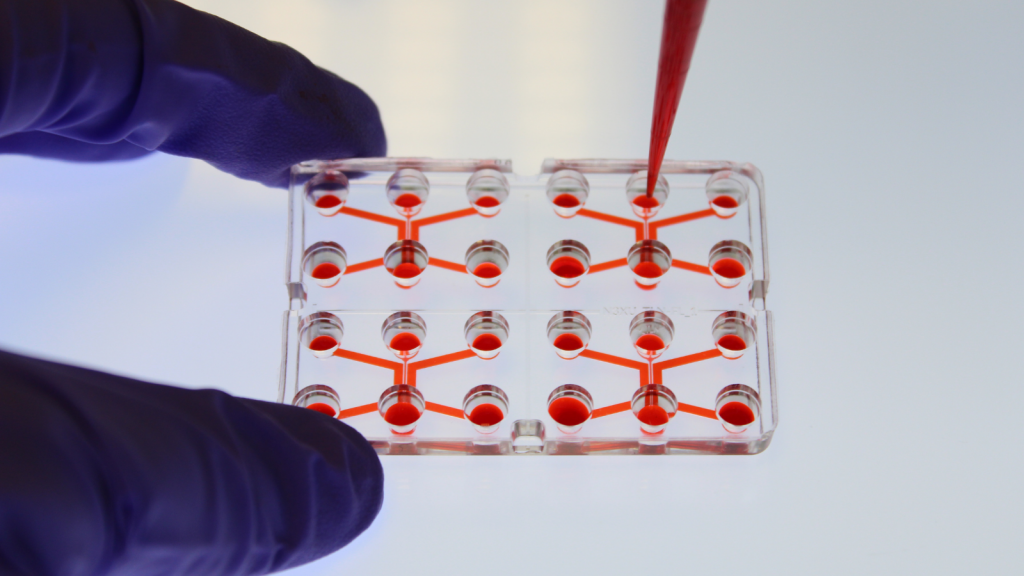
Microfluidics is both the science of manipulating fluids at the micrometer scale (on the order of a thousandth of a millimeter), and the technology of manufacturing miniaturized devices containing chambers and tunnels through which fluids can either flow or be confined.
• Economies of scale , particularly in terms of the quantity of products used (smaller quantities)
• Economies in time spent on experiments (faster)
• Economies on the total cost of the research project (lower cost
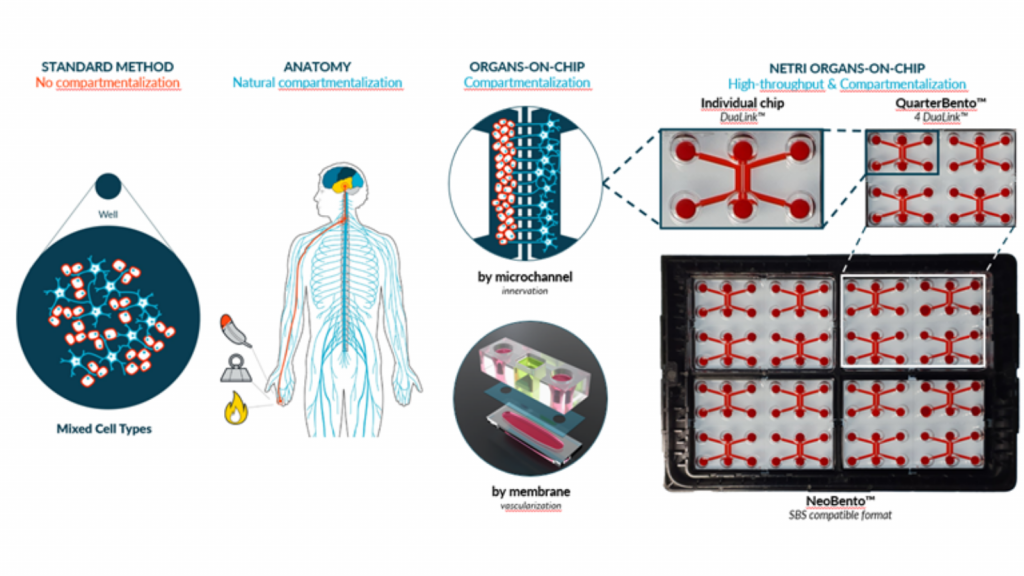
Organs-on-chips (OoCs), also known as microphysiological systems (MPS) or “tissue chips”, are microfluidic devices containing living cells and aiming at recapitulating various functions of organs . Convinced by their potential benefits, the FDA is now inviting drug developers to include non-animal methods , and especially OoCs, in clinical trial authorization submissions . Some governments are even starting to remove the historical legislative roadblocks, such as the USA with the signature of the Modernization Act 2.0 in 2022.
• Mimic various key aspects of human physiology & precise control of the cellular microenvironment
• Upcoming alternative method to animal testing as well as the potential future gold standard to investigate human pathophysiology and future therapeutics
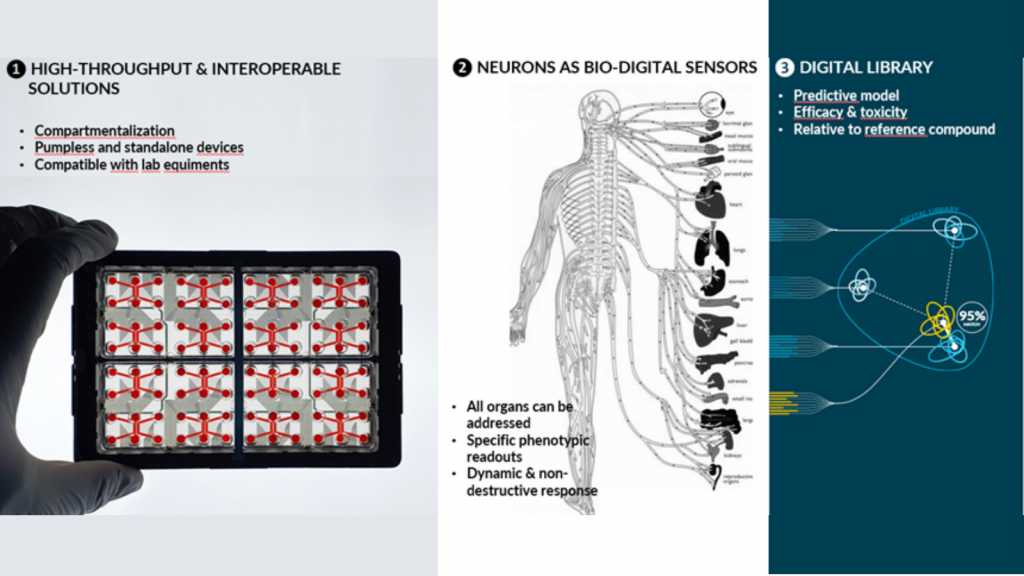
Our goal is to democratize the use of organs-on-chip in order to facilitate their adoption by regulators bodies and healthcare industries. Our approach is to generate the level of data (throughput) and biological completeness (relevance) needed to validate the predictive character in humans based on 3 main axes:
• High-throughput & interoperable devices : compartmentalization, pump-free and compatible with laboratory equipment
• Using neurons as a bio-digital sensors : digital transcription of the functional impact (determination of toxicity or efficacy) of products tested on an innervated target organ, creating a digital signature for each compound tested
• Digital library : comparison of the digital signatures of the compound to be tested with those of compounds already on the market or which have failed in the clinical phase (prediction of toxicity and efficacy)
Discover our new exclusive package
organs-on-chip kits and all our
neuro-organs-on-chip devices.

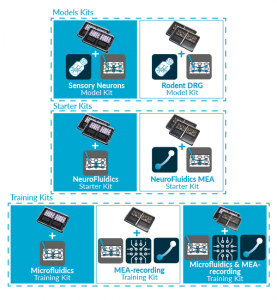
ORGANS-ON-CHIP KITS
Quickly and easily adopt organs-on-chip
into users’ research