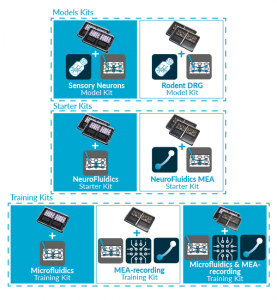
MICROFLUIDICS IS NOW JUST
1-CLICK AWAY WITH NETRI SHOP
Discover our new exclusive package
organs-on-chip kits and all our
neuro-organs-on-chip devices.

Inflammatory Bowel Disease (IBD) modeling
IBD are complex chronic inflammatory disorders of the gastrointestinal (GI) tract. Recent evidence suggests that the gut-brain axis may be pivotal in gastrointestinal and neurological diseases, especially IBD.
We will present the first proof of concept for a microfluidic technology to model bilateral neuro-immunological communication.
We designed a device composed of three compartments with an asymmetric channel that allows the isolation of soma and neurites thanks to microchannels and creates an in vitro synaptic compartment. Human-induced pluripotent stem cell-derived cortical glutamatergic neurons were maintained in soma compartments for up to 21 days. We performed a localized addition of dendritic cells (MoDCs) to either the soma or synaptic compartment. The microfluidic device was coupled with microelectrode arrays (MEAs) to assess the impact on the electrophysiological activity of neurons while adding dendritic cells. The data highlight that an electrophysiologic signal is transmitted between two compartments of glutamatergic neurons linked by synapses in a bottom-up way when soma is exposed to primed dendritic cells.
In conclusion, this study authenticates communication between dendritic cells and neurons in inflammatory conditions such as IBD. This platform opens the way to complexification with gut components to reach a device for pharmacological compound screening by blocking the gut-brain axis at a mucosal level and may help patients.
Alzheimer's Disease (AD) modeling
Mounting evidence suggests that oligomeric forms of amyloid beta (AβO) and Tau (TauO) play significant roles in the pathophysiology of AD. Current preclinical models often fail to accurately predict human physiological and pathological conditions. Therefore, it is crucial to develop novel testing methods to enhance the reliability of drug efficacy predictions. Microfluidic devices, in conjunction with human-induced pluripotent stem cells (hiPSCs), hold immense potential to transform the drug screening process by bridging the translational gap between animal models and human physiology.
We will introduce a thoroughly characterized microfluidic-based hiPSC culture designed to evaluate the impact of misfolded protein oligomers in inducing AD. We employed a compartmentalized co-culture of hiPSC-derived glutamatergic and GABAergic neurons utilizing NETRI's DuaLink MEA microfluidic device (3 compartments for isolating soma and neurites via microchannels). This setup was combined with microelectrode arrays (MEAs) to assess electrophysiological activity. Misfolded human oligomers, produced by ETAP-Lab, were introduced into a primary rodent cells culture in NETRI's DuaLink Shift MEA, (3 compartments for isolating soma, neurites, and synaptic connections via microchannels) at various stages and concentrations. The effects of these oligomers on axonal degeneration, cell viability, expression of phenotypic markers, synaptic alterations, and electrophysiological activity were comprehensively analyzed.
By leveraging NETRI's multidisciplinary expertise in engineering, biology, and digital technologies along with ETAP-Lab's proficiency in neurological modeling and oligomer production, we have established a brain-on-chip model of AβO and TauO injuries. This model can be utilized for drug screening aimed at developing new therapeutics for AD. This highlights the localized axonal degeneration induced by acute oligomer exposure in a dose-dependent manner within the axonal compartment, without evidence of neurodegeneration in the somatic compartment. This marks a significant advancement in the establishment of highly relevant and standardized AD models, which serve as invaluable resources for both basic research and pharmaceutical drug discovery. These models facilitate the elucidation of AD's pathological mechanisms and enable the screening of novel and effective treatments.
Funding for this project is provided by the French Government under the "France 2030" call for proposals for "Innovations in Biotherapies & Bioproduction."
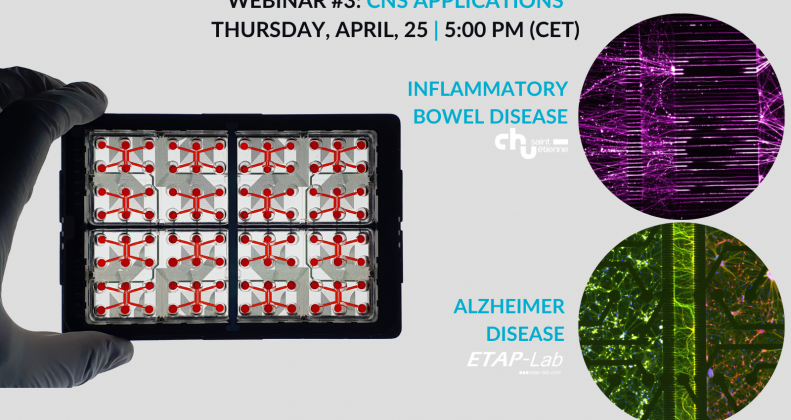
• Use Case #1: Inflammatory Bowel Disease modeling, in collaboration with CHU Saint-Etienne
• Paves the way for complexing with intestinal components
• Use Case #2: Alzheimer's disease modeling, in collaboration with ETAP-Lab
•Paves the way for assessment of axonal loss, an early event in Alzheimer's Disease that precedes neuronal death
• Q&A Session

Benoît Maisonneuve is the Clinical Translation Product Owner of NETRI. Holding a dual Ph.D. in rheology and bioengineering, he has been a pioneer in various technologies, ranging from brain-on-chip to modeling age-related diseases, as well as the production of 3D hydrogels and CRISPR/Cas9 strategies. His professional background in the industry includes managing clinical trials and pharmaceutical projects (for drugs in clinical trials and commercial specialties). Benoît has held key positions within international committees and standardization commissions, providing valuable expertise to this industry. Benoît joined NETRI in 2021 as a scientist and is committed to advancing science and technology. Convinced that NETRI's innovations will shape the future of testing in various fields, he has been serving as the Clinical Translation Product Owner since 2022.
Discover our new exclusive package
organs-on-chip kits and all our
neuro-organs-on-chip devices.

ORGANS-ON-CHIP KITS
Quickly and easily adopt organs-on-chip
into users’ research