MICROFLUIDICS IS NOW JUST
1-CLICK AWAY WITH NETRI SHOP
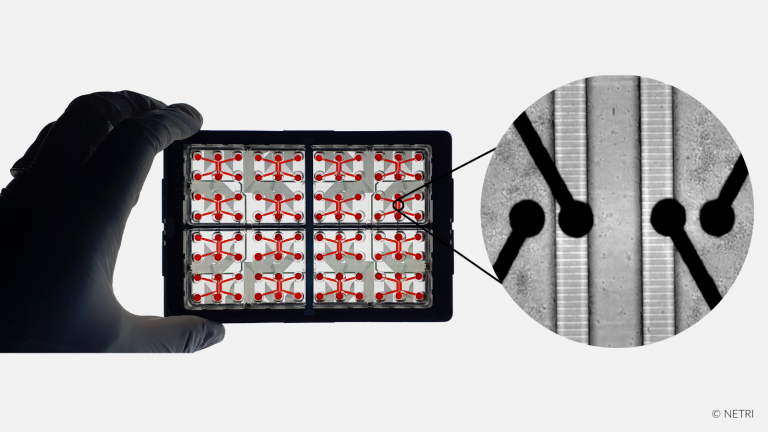
Discover our new exclusive package
organs-on-chip kits and all our
neuro-organs-on-chip devices.

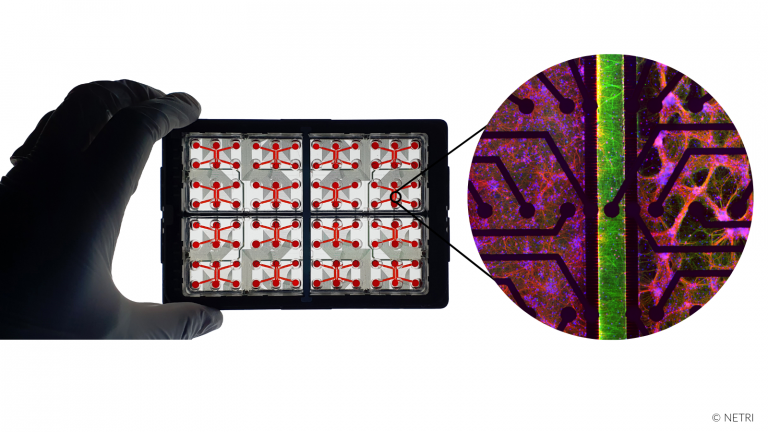
Recreate the compartmentalized innervation or irrigation of skin in organs-on-chip microfluidic devices.
• Innervated skin 2D
• Irrigated skin 2D
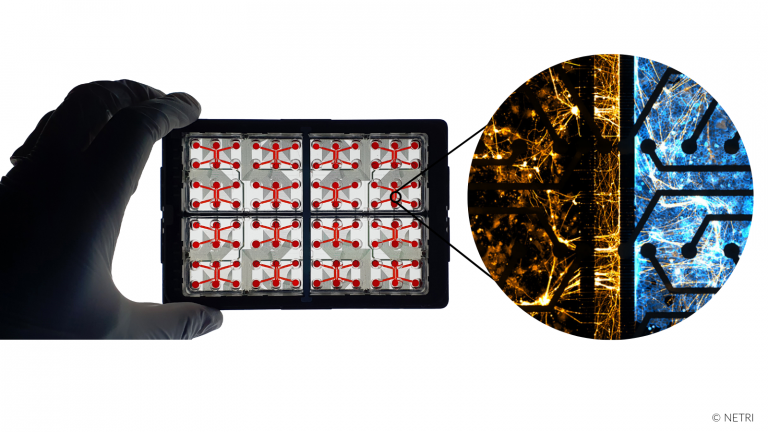
Recreate the compartmentalization of Peripheral Nervous System (PNS) in organs-on-chip microfluidic devices.
• Directional Sensory Neurons Projections
• Directional Motor Neurons Projections
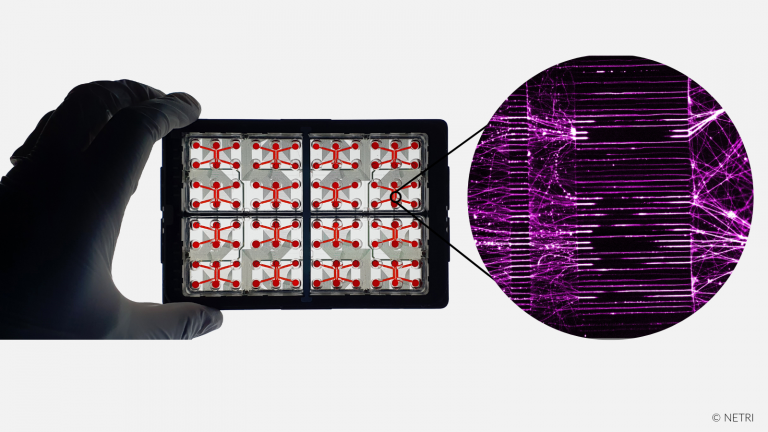
Recreate the compartmentalization of Central Nervous System (CNS) in organs-on-chip microfluidic devices.
• Compartmentalized GABAergic & Glutamatergic Neurons
• Compartmentalized Glutamatergic & Glutamatergic Neurons
• Compartmentalized Cortical & Hippocampal Neurons
• Perfused Cerebral organoid
Find out more about the models currently under development and their associated readiness level.
• Compartmentalized Dopaminergic & GABAergic Neurons
• Compartmentalized Muscle Cells & Motor Neurons
• Compartmentalized Cortical & Striatum Neurons
• Dorsal Root Ganglion
• Multi-organs
• Neurons-to-X
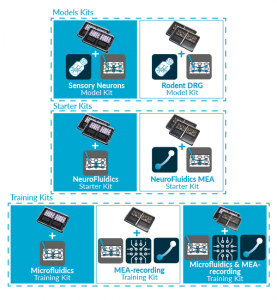
Discover our new exclusive package
organs-on-chip kits and all our
neuro-organs-on-chip devices.

ORGANS-ON-CHIP KITS
Quickly and easily adopt organs-on-chip
into users’ research