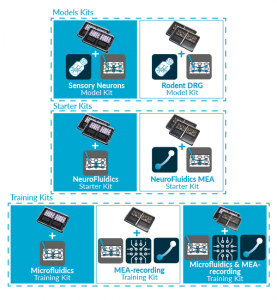
MICROFLUIDICS IS NOW JUST
1-CLICK AWAY WITH NETRI SHOP
Discover our new exclusive package
organs-on-chip kits and all our
neuro-organs-on-chip devices.

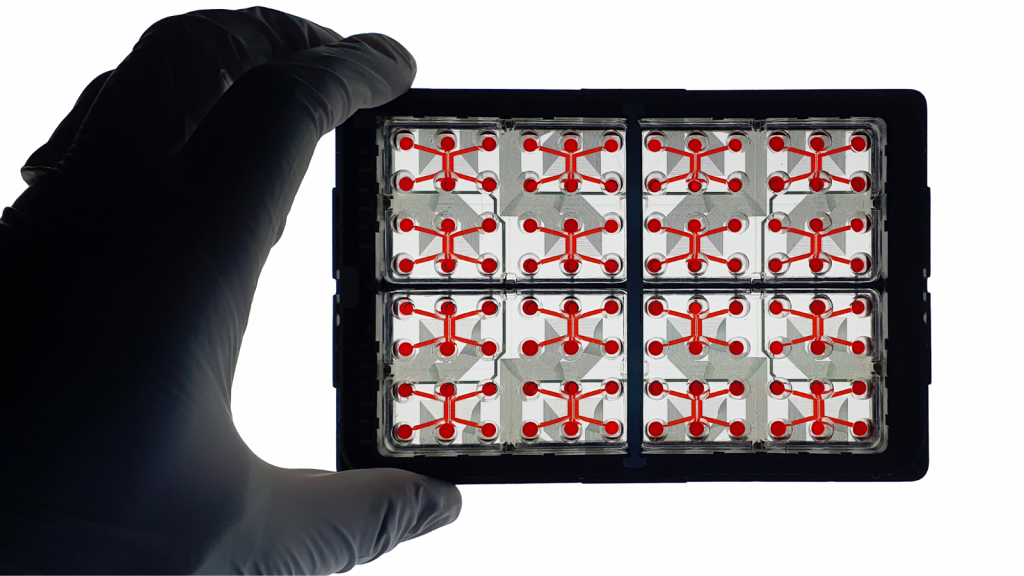
• Up to 16 data points
• 2 compartments for independant culture per chips
• 1 compartment for synapses isolation
• Microchannels technology of different lenghts to monitor growth kinetics & create synaptic compartment
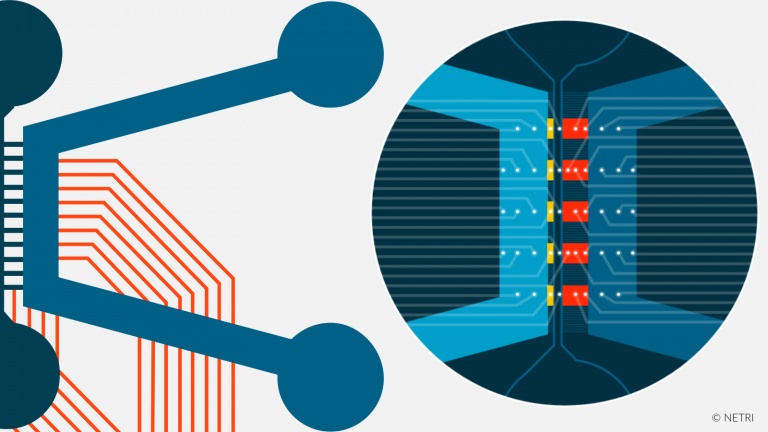
Each chips are compartmentalized by microchannels technology. This enebles the creation of axodendritic synapses in the 2nd channel and the monitoring of axonal growth kinetics. Microchhanels technology enables also co-culture of different cell types (GABAergic neurons & glutamatergic neurons…). By adding the MEA option, record the electrical activity of synapses, isolated per compartment.
Specially designed to monitor the functional activity of synapses.
• 16 microfluidic chips per NeoBento™ MEA PRO with 672 electrodes
• 8 microfluidic chips per NeoBento™ MEA EDGE with 336 electrodes
• Synapses electrophysology activity isolation per compartment & remote stimulation
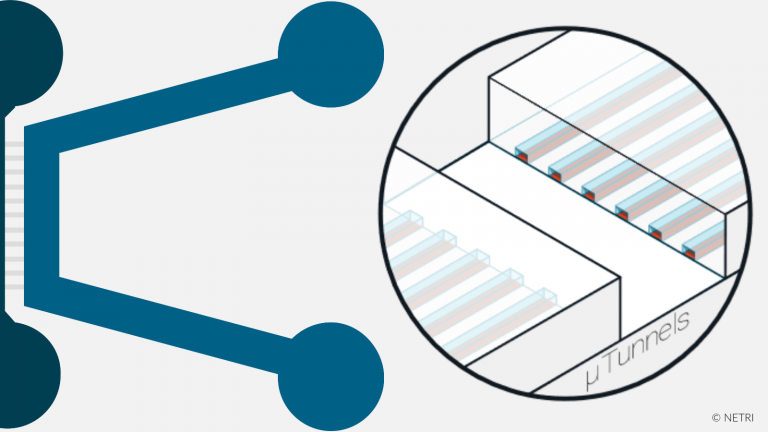
Specially designed to recreate synapses isolation and monitor axonal growth kinetics.
• 16 microfluidic chips per NeoBento™ FULL
• 8 microfluidic chips per NeoBento™ LIGHT
• Discontinious connectivity
• Asymmetrical shape with microchannels of different length (shorter length between the first and second channel than between the second and third channel).
NeoBento™, the standard format for NeuroFluidics Devices chips, available up to 4 QuarterBentos™ (up to 16 chips).
• Standard ANSI format (96-well plate)
• Pump-free & expensive equipment-free
• Standard equipment (liquid handling & imaging) compatibility
Compartmentalized microfluidics devices to co-culture 2 different cell types.
• Each compartment has its own media & coating solutions
• Discrimination of mode and mechanism of action
• Synaptic transmission & localization
In-depth reading of the data to better understand the study results and potential implications.
• Electrophysiological recording (MEA)
• Imaging (Immunofluorescence, Calcium Imaging…)
• Biochimic analysis (ELISA, Lysis cells analysis, Liquid Chromatography…)
Microchannels technology allow synaptic compartmentalization (pre-, post- and synaptic compartments).
• Neuroinflammation
• Inflammatory Bowel Disease
• Huntington’s disorders
• 3 architectures : DuaLink MEA, DuaLink Shift MEA & TriaLink MEA
• 8 or 16 data points per plate
• Training & Organs-on-chip Kits
• Electrical neurons activity recording
• 3 architectures : DuaLink, DuaLink Shift & TriaLink
• 8 or 16 data points per plate
• Training & Organs-on-chip Kits
• Imaging & Biochimic analyses readouts
Discover our new exclusive package
organs-on-chip kits and all our
neuro-organs-on-chip devices.

ORGANS-ON-CHIP KITS
Quickly and easily adopt organs-on-chip
into users’ research